Abstract
Introduction:
Multiple myeloma (MM) is the second most common hematologic malignancies in the world. New approved therapeutic agents have improved the survival rates of myeloma patients. However, it is often unclear what is the best treatment in different settings, and the treatment cost is rising. In this study, we assess the way MM is treated in a developed country in Asia.
Methods:
A retrospective study of newly diagnosed MM patients from June 2009 to April 2017 from Singapore was conducted. The analysis focused on the age of patient, medication regimes used for different lines of treatment, whether autologous stem cell transplant (SCT) was received and the estimated cost of treatment. Treatment free period (TFP) was measured as time from the end date of previous treatment to the start date for the next treatment. The time to next treatment (TTNT) was measured from the start date of the first treatment to the start date of the next line of treatment.
Results:
A total of 143 patients were included in the analysis. Among the 96 (67.1%) patients under 65 years old, only 73 (76%) received autologous SCT treatment. Overall, 56 (39.2%) of the patients progressed and received second (2nd) line treatment, while 21 (14.7%) and 9 (6.3%) received 3rd and 4th line treatment respectively. The most frequently used drugs for 1st line treatment were Bortezomib (75.5%), Dexamethasone (87.4%), Thalidomide (63.6%) and Cyclophosphamide (53.8%). This reflects that the 2 most commonly used regimens for 1st line treatment are VCD and VTD. For 2nd line treatment, Lenalidomide (50%) and Dexamethasone (94.6%) were the main drugs used, reflecting that RD was the predominant regimen used in this phase. For 3rd line treatment, Dexamethasone (100%), Pomalidomide (38.1%), Cyclophosphamide (33.3%) and Lenalidomide (33.3 %) were the main drugs used.
Focusing on individual drugs, while Lenalidomide was only used 7.7% of the time for 1st line treatment, usage increased to 50% and 33.3% for 2nd and 3rd line treatment respectively. For Pomalidomide, while usage was low at 0% and 7.1% for 1st and 2nd line treatment respectively, it increased to 38.1% for 3rd line treatment. Bortezomib, Thalidomide and Cyclophosphamide were predominantly used as 1st line treatment choices, with usage rates of 75.5%, 63.6% and 53.8% respectively. Their usage rates were significantly reduced in 2nd and 3rd line treatment.
The average treatment cost per TFP (weeks) among 56 patients who progressed to 2nd line treatment was $673.81/week. The average cost increased to $2,239/week and $11,556.56/week for the patients who received 3rd and 4th line treatment respectively. For SCT patients (30 patients progressed to 2nd line, 7 patients to 3rd line, and 4 patients to 4th line), the average cost per TFP was $705.87/week, $2,078.66/week and $35,854.20/week for 2nd, 3rd and 4th line of treatment respectively. For non-SCT patients (26 patients progressed to 2nd line, 14 patients to 3rd line, and 5 patients to 4th line), the average cost per TFP was $603.64/week, $2,333.25/week and $5,959.72/week for 2nd, 3rd and 4th line of treatment respectively.
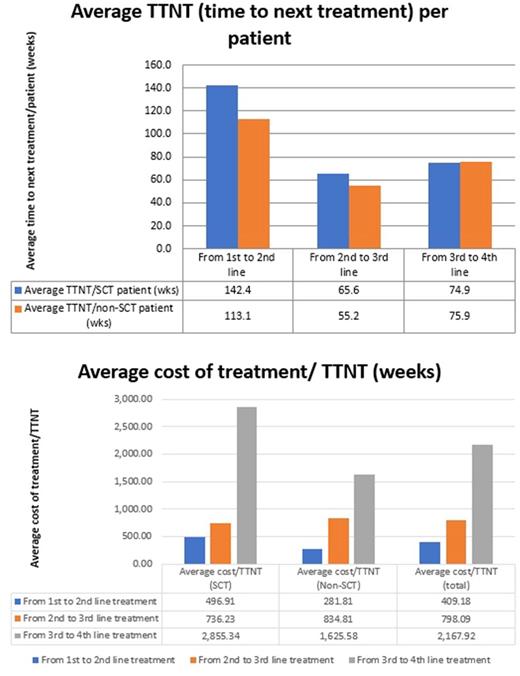
The average TTNT per patient for 1st, 2nd and 3rd treatment line progression was 128.8, 58.7 and 75.5 weeks respectively. The average TTNT per SCT patient was 142.4, 65.6 and 74.9 weeks for 1st, 2nd and 3rd treatment line progression respectively, as compared to 113.1, 55.2 and 75.9 weeks for non-SCT patients. Overall, the average treatment cost per week of TTNT was $409.18, $798.10 and $2,167.92 for 1st, 2nd and 3rd treatment line progression respectively - the average cost for SCT patients was $496.91, $736.23 and $2,855.34, while the average cost for non-SCT patients was $281.81, $834.81 and $1,625.58 respectively.
Conclusions:
Treatment for multiple myeloma remains expensive. While the benefits of subsequent lines of treatment decreases, the average treatment cost increases. With the increasing availability of expensive but yet more effective treatments, these could be incorporated early into the treatment regimen to maximise therapeutic benefit. Despite the higher cost of these drugs, cost-effectiveness could be improved, or at least remain similar if TTNT can be greatly prolonged.
Chng: Janssen China R&D: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal